22-Nov-2011
The GRAVITEL Process – Introduction
The GRAVITEL or Gravity for Electroplating process was developed by Maschinenfabrik Andritz (Ruthner Division) in Vienna, Austria at the beginning of 1980. The first large line was started in September’85 at Voestalpine in Linz, Austria. At present there are about ten high capacity electro galvanizing lines (EGL) around the world using GRAVITEL system successfully. It is estimated that by 2010, the installed capacity of GRAVITEL will exceed 4 million tons (MT) per year. The GRAVITEL process is carried out in a vertical cell, where the strip is running at a narrow distance between two movable anode boxes. The anodes are made of titanium sheet coated with conductive iridium oxide. The electrolyte then flows into the gap between anode and strip and is accelerated by gravity up to a speed of 5 meters/sec. High electrolyte flow enables high capacity electroplating at a current density up to 180 amp per square decimeter.
Fig: GRAVITEL cell
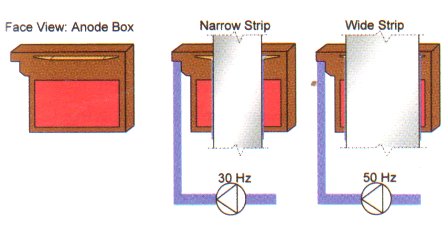
Fig: GRAVITEL - Electrolyte flow controlled by Frequency controlled Pump
The vertical GRAVITEL cell has high flexibility according to the width, shape of the steel strip and the plating mode i.e. one or two side coating. The variation of the strip width is adjusted by the electrolyte flow in the anode boxes, which is set by frequency controlled pumps. The GRAVITEL process is further characterized by even coating distribution; no edge masks are required since no edge over coating occurs. Each cell has 2 pair of anodes which can be engaged or disengaged hydraulically. In the engaged position the anode gap can be easily varied according to the waviness of the strip. The narrow gap between the anodes and the strip results in low ohmic loss in the electrolyte which leads to low energy consumption. While coating only one side, the electrolyte flow to the inner anode boxes is stopped and the inner anode boxes are moved away from the strip. Such operations are performed automatically and sequentially as the weld seam moves through the plating cells without interrupting the production or causing any strip loss. The plating current is transferred from the rectifier to the steel strip via the conductor roll. The contacting surface of the conductor roll is made of high duty alloys. In order to get the highest surface quality of the coated strip each conductor roll is rinsed in a tray and polished by an oscillating scraper. A closed loop cooling circuit is provided from inside to cool the conductor rolls.

Fig: GRAVITEL- Automatic Anode adjustment for Strip Waviness
Zinc Coating (Galvanizing)
The GRAVITEL process uses a sulfate electrolyte in which zinc is plated at the cathode (the strip). The electrochemical reaction that takes place is –
Zn2+ + 2e– → Zn
At the anode acid ions and oxygen are formed –
H2O → 2H+ + ½ O2 + 2e–
The cathode current efficiency is high around 97%, the remainder being hydrogen evolution reaction. For each equivalent of zinc plated, exactly one equivalent of acid is generated, which can in turn be used to dissolve one equivalent of zinc in the dissolving station, according to the following reaction –
Zn + 2H+ → Zn2+ + H2
The hydrogen evolved as above is diluted by air to a safe level well below the lower explosion limit of hydrogen-air mixture. The hydrogen level in the off-gas of the dissolving station is constantly monitored by a hydrogen detector. From the above equations it can be seen that maintaining the proper bath chemistry is also easy because the plating-dissolving process is actually self-regulating. Thus the GRAVITEL process of electro galvanizing is more advantageous than the systems with soluble anodes where the anodic dissolution efficiency is generally higher than cathode plating efficiency, which leads to a surplus of metals in the electrolyte. This is balanced by the partial use of insoluble anodes or (especially in the case of chloride electrolytes) by bleed-off of considerable quantities of electrolyte.
GRAVITEL process of Electro Galvanizing
|
Process speed
|
Up to 180 m/minute
|
Current efficiency
|
97%
|
Electrolyte velocity
|
Up to 5 m/sec
|
Electrolyte flow / cell
|
Up to 1000 m3/h
|
Current density
|
Up to 180 A/dm3
|
Anodes
|
Insoluble Ti anodes with IrO2 coating
|
Anode – strip gap
|
8 mm
|
GRAVITEL: Process steps
To improve the flatness of the strip first it is passed through a tension leveler. This is required to run the anodes in the GRAVITEL cells in “narrow position” which enables an energy saving production and assures best coating distribution over the strip width. The strip then enters the chemical degreasing section where oil, grease and other similar particles present, if any, on the strip are removed from the surface in order to prepare it for subsequent coating. The cleaning is done by spraying hot alkaline solution onto both sides of the strip. An additional cleaning is also done by means of mechanical brushing of the strip. Further, a vertical electrolyte degreasing cell ensures that all the particles have been removed from the surface of the strips. The process is based on the central conduction system, i.e. there is no conductor rolls used as the current is applied directly to the strip via the electrolyte and also discharged likewise. When a strip is passing through a pair of cathodes, the strip surface will be polarized as anode and vice-versa. Hydrogen and oxygen are evolved respectively, on the strip surface resulting in a high cleaning effect. The degreasing section is followed by a counter current 3 -stage rinsing section to remove remaining cleaner solution from the strip. The first rinsing stage is executed as scrub brush machine for additional mechanical cleaning of the strip surface. Thereafter the cleaned strip proceeds to the pickling section where the strip surface is activated for the subsequent plating. This section consists of a vertical cell of the flooded type with dilute sulfuric acid as pickling agent. A 3 – stage rinsing section assures a clean strip for the galvanic process in the GRAVITEL cells. The actual plating takes place in a number of GRAVITEL cells as above.
Electrolyte Circulation System
All GRAVITEL cells are connected to a big electrolyte circulation tank. Besides, there are various other circulation systems. The enrichment of the electrolyte with zinc proceeds in a conical tank filled with zinc granules – the zinc dissolving section. The electrolyte is brought upwards through the granules and overflows the dissolving tank into the settling tank where trapped hydrogen can escape from the liquid. The rate at which zinc dissolves depends on many factors such as electrolyte temperature, sulfuric acid content and its rate of flow through dissolving tank. The pump which controls the electrolyte flow to the dissolving station is equipped with variable speed drive in order to adapt the dissolving intensity to the actual zinc content of the electrolyte determined by total current consumption in the plating cells and by means of electrolyte analysis. The liberated hydrogen content in the exhausted air is monitored to avoid any danger. If the hydrogen level reaches the first limit the speed of the dissolving pump is reduced to the minimum. Then the dissolving station is drained and flushed with pure water to stop the hydrogen evolution. To separate the accumulated water from the electrolyte – mainly from the spraying pipes at the conductor rolls – a flash evaporator is installed which consists of two stages and operates at a low absolute pressure. The electrolyte heating and cooling is integrated in the evaporator control. Here the joule’s heat from the plating process is used for the evaporation, simultaneously cooling the electrolyte. The achieved distillate quality allows reusing the water as ‘demineralised’ water in the rinsing sections of the line. Thus the zinc electrolyte is never exchanged and is operating in a closed loop with zero effluents.
After the plating process is finished, the strip is cleaned in a 4-stage rinsing section. The first stage can be operated as acidic rinse where the acid concentration is adjusted by a conductivity control loop; the three following stages are operated as cascade rinsing.
GRAVITEL: Process Control and Post Treatment
The process solution section is fully automatically controlled by Andritz Line Master (ALM-EGL). Based on primary coil data and the requested coating weight the coating algorithm calculates the set-point for process speed and rectifier current for each cell continuously in order to maximize the output of the electro galvanizing lines. A closed loop process control corrects the calculation based on the online measured zinc dissolving pump.
There are various possibilities of post treatment which can be done according to the customer requirement and application. The following post treatments are possible in an electro galvanizing line –
- Phosphatizing
- Anti-fingerprint coating
- Passivation
- Pre-lubrication coating
- Oiling
In case of phosphatizing the layer is applied by spraying. The surface of the strip must be activated for the subsequent phosphatizing. The phosphate layer serves as a lubricant for deep drawing and a basis for later applied paint. The phosphatizing solution is circulated through a bag filter and is heated up by a heat-exchanger which is operated with hot water. This operation is followed by a 3-stage rinsing section.
Antifingerment, passivation or pre-lubrication coatings are typically applied in a roll coater. In a roll-coater, the top and bottom side of the galvanized strip are coated with the respective liquid chemical. In order to achieve the required coating thickness the applicator and the pick-up rolls are driven by speed controlled motors and adjusted automatically. The roll coater is followed by a strip drier and an air-cooling section. In the exit portion of electro galvanizing line, an electrostatic oiler allows an application of protective oil on the finished (coated) strip.
Gravitel Process: Advantages and Applications
With more than ten operating plants and more under construction, the GRAVITEL system is the most widely used electro galvanizing process worldwide. The key benefits are highest surface quality, even coating distribution, high capacity electroplating and high flexibility in terms of strip width adjustment and single or double sided coating, energy- and zinc- saving operation with minimum amount of downtime. GRAVITEL process has got applications for electro-galvanizing of automotive exposed sheet, household and office appliances like air conditioners, microwaves, washing machines, flat TVs, computers etc. Important players in steel industry from America, Asia (especially China), and Europe chose the GRAVITEL process from Andritz AG for their new electro galvanizing lines.