MgO-CaO Phase Diagram
Fig: MgO-CaO binary Phase Diagram
The above figure shows the phase diagram of MgO-CaO binary system. The solid solubility at high temperatures leads to formation of a high temperature bond in dolomite refractories.
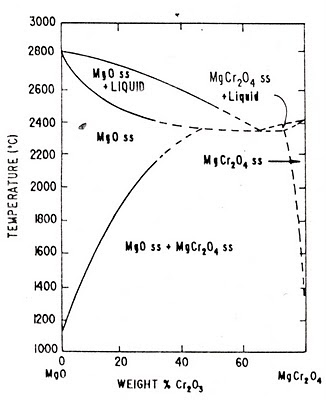
MgO-Cr2O3 System (MgO-MgCr2O4 Phase Diagram)
Fig: MgO-MgCr2O4 binary Phase Diagram
The only intermediate compound which exists in the binary system MgO-Cr2O3 is Magchrome spinel (MgO.Cr2O3 or MgCr2O4). From the above phase diagram of Magnesite-Magchrome spinel (MgO-MgCr2O4) binary system it can observed that for steel plant refractories direct bonding between magnesia-chrome phase is formed when these two are heated together at temperatures above 1600OC as a result of the partial solubility of the constituents.
Dissolution Kinetics of Refractory Oxides
Refractories are non-metallic materials used for the lining of kilns and furnaces required for high temperature operations in several metallurgical and non-metallurgical industries such as iron & steel, aluminium, copper, glass, cement, petrochemicals etc.
Dissolution of solid oxides in liquid slag is governed mainly by –
(1) Chemical reaction at the slag / refractory interface,
(2) Transport or diffusion of reacting species.
In the second case, rate of dissolution can be expressed in terms of Nernst equation:
J = D (Cs – Cm) / ∂
Where, D is the diffusion coefficient (m2 s–1), Cm and Cs are, respectively, concentration and saturation solubility of refractory in slag (g m–3), and ∂ is the effective boundary layer thickness (m). Increasing D or decreasing ∂ (i.e. increasing D/∂) result in increase of dissolution rate, J. Moreover, it is also clear from the above equation that the value of (Cs – Cm) strongly influences the dissolution rate. If slag is saturated with refractory oxide then J = 0. Naturally, to minimize rate of dissolution, it is necessary to minimize (Cs – Cm). For example, with increasing MgO content in the slag, the corrosion of the periclase phase in Mag-Chrome refractories will decrease. If Cm = 0, then the value of (Cs – Cm) reaches a maximum and thus, so does the dissolution rate.
Dissolution kinetics of MgO-CaO and Magnesite-chrome refractories in secondary steel slag was studied by Chen Zhaoyou Wu Xuezhen Ye Fangbao at Luoyang Institute of Refractories Research by means of the rotating cylinder method [See details]. Materials investigated include four MgO-CaO samples (MgO content: 40 to 93%) and two magnesite-chrome samples (co-clinkered and semi-rebonded). The experiments were carried out in Argon atmosphere at different temperatures (1600-1750OC) and revolution speeds (200 to 500 rpm) using synthetic slags similar to VOD and AOD slags with different basicities (0.6-2.68). Based on their experimental results the mechanism and kinetics of the dissolution process are discussed. The conclusions drawn are as follows:
1. Erosion resistance of magnesite-chrome (MgO-Cr2O3) refractories is better than that of MgO-CaO materials.
2. When the content of MgO is about 60-80%, slag dissolution resistance of MgO-CaO samples will be comparatively higher. When basicity of slag is 1.0, the dissolution rate of magnesite-chrome refractories (i.e. Mag-Chrome, MgO-Cr2O3) is much lower than that of MgO-CaO.
3. With increase of basicity of slag, the dissolution rate of magnesite-chrome increases, whereas that of MgO-CaO decreases.
4. For the increase of temperature of 100OC at one time, the dissolution rate of MgO-CaO increases by 2-3 times while that of magnesite-chrome increases 5-6 times. Dissolution activation energy for MgO-CaO refractory is 70 kcal/mol and that for magnesite-chrome is 110 kcal/mol. The diffusion coefficient of MgO in the slag is 3.7×10~(-5)cm~2/s.
5. M_2S is formed by magnesite-chrome with acid slag in the reaction zone while C_2S is formed by MgO-CaO with basic slag in the reaction zone.
6. The process of dissolution of MgO-CaO refractories in slag is controlled by the diffusion mechanism.